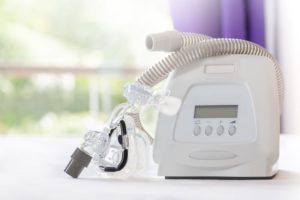
Philips Reaches $1.1B Settlement Over Defective CPAP Machines

The settlement, announced in conjunction with the company’s first-quarter earnings report, assigns a total payout for personal injury litigation resulting from the 2021 recall of certain Philips Respironics devices used to treat sleep apnea and other breathing conditions. The deal also earmarks funds for medical monitoring of people who used the defective devices.
The 2021 recall came amidst disturbing reports that the machines were emitting toxic chemicals stemming from the breakdown of noise abatement foam. As part of the settlement, Philips did not admit fault, nor did it acknowledge that any injuries or deaths were caused by the company’s devices.
The settlement comes three weeks after Philips Respironics was handed stiff penalties that limit its ability to do business in the United States. On April 9, a U.S. federal court issued a consent decree that bans the company from selling sleep apnea machines in the U.S. and halts production until a number of requirements have been met. Philips must also hire independent safety experts to oversee the process and submit to facility inspections for five years.
The move is a crucial step in ensuring that more consumers aren’t sold dangerous devices. Last year, an investigation by ProPublica and the Pittsburgh Post-Gazette found that Philips had been fielding complaints about its devices for more than a decade but had kept this information from the public.

Thousands of people who used the defective machines reported severe side effects, including respiratory ailments, cancer, and liver and kidney diseases. Since April 2021, the Food and Drug Administration has received more than 500 reports of deaths allegedly associated with the recalled devices.
Furthermore, the replacement machines that Philips issued were found to pose health risks as well, as they were made with a silicone-based foam that was found to emit toxic chemicals. Though Philips insists that the new machines are safe, the FDA said that more testing is needed. Under the new consent decree, an independent safety monitor will investigate the quality of the prior testing and may require Philips to do additional testing on the foam.
The decree also lays out remediation measures for affected Philips customers, including the option of a partial refund. In a press release, Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, notes that it “marks the first time a device company is providing a remediation payment option for a recalled device under a consent decree.”
There are a few exceptions in the decree. Philips can continue to sell a select group of devices that the FDA deems “medically necessary,” but it must give a percentage of the revenue to the U.S. government. The company can also continue selling devices outside the U.S.
Philips Respironics, which is based outside Pittsburgh, has a long history in the sleep apnea industry. Formerly known as just Respironics, it produced the first commercially available CPAP machines in 1985. Philips acquired the company in 2008.
Several years later, the company redesigned its CPAP machines to add industrial foam made of polyester-based polyurethane. The intent was to muffle the sounds the machine made, but the foam crumbled when exposed to heat and humidity. Soon after, customers began reporting concerning symptoms.
It’s unclear when, if ever, Philips will resume producing sleep apnea devices for the U.S. market. If it does, it will face the challenging task of regaining consumer trust and reassuring the public that its products are safe.
Got a hot tip? Pitch us your story idea, share your expertise with SleepFoundation.org, or let us know about your sleep experiences right here.
References
9 Sources
-
Koninklijke Philips N.V. (2024, April 29). Philips delivers first-quarter results in line with 2024 performance improvement plan; Resolves the Respironics personal injury and medical monitoring litigation in the US for USD 1.1 billion.
https://www.philips.com/a-w/about/news/archive/corpcomms/news/press/2024/philips-first-quarter-results-2024.html -
U.S. Department of Justice, Office of Public Affairs. (2024, April 9). Court enjoins Philips Respironics from manufacturing and distributing adulterated and misbranded sleep and respiratory devices at or from three Pennsylvania facilities.
https://www.justice.gov/opa/pr/court-enjoins-philips-respironics-manufacturing-and-distributing-adulterated-and-misbranded -
Cenziper, D., Sallah, M. D., Korsh, M., Robinson-Johnson, E., & Sager, M. (2023, September 27). Philips kept complaints about dangerous breathing machines secret while company profits soared. ProPublica. Pittsburgh Post-Gazette.
https://www.propublica.org/article/philips-kept-warnings-about-dangerous-cpaps-secret-profits-soared -
U.S. Food and Drug Administration. (2024, January 31). Problems reported with recalled Philips ventilators, BiPAP machines, and CPAP machines., Retrieved April 29, 2024, from
https://www.fda.gov/medical-devices/recalled-philips-ventilators-bipap-machines-and-cpap-machines/problems-reported-recalled-philips-ventilators-bipap-machines-and-cpap-machines -
U.S. Food and Drug Administration (2021, November 12). FDA provides update on recall of certain Philips Respironics breathing assistance machines., Retrieved April 29, 2024, from
https://www.fda.gov/news-events/press-announcements/fda-provides-update-recall-certain-philips-respironics-breathing-assistance-machines -
Philips Respironics. (n.d.). Explained: Silicone sound abatement foam used in DreamStation 2 and the sleep and respiratory care devices remediated as part of the June 2021 Philips Respironics recall., Retrieved April 29, 2024, from
https://www.philips.com/a-w/about/news/archive/standard/news/articles/2023/explained-silicone-sound-abatement-foam-used-in-dreamstation-2-and-the-sleep-and-respiratory-care-devices-remediated-as-part-of-the-june-2021-philips-respironics-recall.html -
U.S. Food and Drug Administration. (2024, April 9). Federal court enters consent decree against Philips Respironics following recall of certain sleep therapy machines., Retrieved April 29, 2024, from
https://www.fda.gov/news-events/press-announcements/federal-court-enters-consent-decree-against-philips-respironics-following-recall-certain-sleep -
Respironics. (2007, December 20). Respironics, Inc. fact sheet., Retrieved April 29, 2024, from
https://www.newscenter.philips.com/pwc_nc/main/shared/assets/2007_pressreleases/20071217_respironics/Respironics_Corporate_Fact_Sheet_FINAL.pdf -
Royal Philips Electronics. (2008, March 14). Philips announces completion of tender offer to acquire Respironics [Press release]., Retrieved April 29, 2024, from
https://www.sec.gov/Archives/edgar/data/313216/000095012308002956/y51433a9exv99waw2wi.htm